Ketogenic nutrition is a nutrition-therapeutic approach to support conventional cancer treatment. The outstanding feature of this nutritional therapy is that it specifically impacts the metabolic properties of cancer cells and exploits the “weaknesses” of these metabolic changes.
Cancer cells absorb enormous quantities of sugar in the form of glucose (grape sugar) compared to the surrounding tissue to secure their increasing energy requirement for growth and reproduction. However, while most healthy cells “burn up” nutrients, many cells, especially aggressive, metastatic and therapy-resistant cancer cells show an unusually high rate of fermentation of sugar, even in the presence of oxygen [note]Warburg, O. (1956): On the origin of cancer cells. Science 123 (3191): 309-314. [Link to the article].[/note]. Recent studies could clarify that the cancer cells, in switching over to this alternative energy source, protect themselves against oxidative stress [note]Aykin-Burns, N. et al. (2009): Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 418 (1): 29–37. [Link to the article].[/note] [note]Spitz et al. (2000): Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci 899: 349–362. [Link to the article].[/note]. At the same time, by doing this, they inhibit the efficiency of many chemotherapy drugs and radiation forms. On the basis of these findings, the concept evolved to deprive cancer cells of the important energy suppliers through a significant reduction in sugar intake, for example to increase oxidative stress in the tumour cell [note]Allen, B. G. et al. (2013): Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res 19 (14): 3905–3913. [Link to the article].[/note] [note]Fath, M. A. et al. (2009): Mitochondrial electron transport chain blockers enhance 2-deoxy-D-glucose induced oxidative stress and cell killing in human colon carcinoma cells. Cancer Biol Ther 8 (13): 1228–1236. [Link to the article].[/note] [note]Hadzic, T. et al. (2010): Paclitaxel combined with inhibitors of glucose and hydroperoxide metabolism enhances breast cancer cell killing via H2O2-mediated oxidative stress. Free Radic Biol Med 48 (8): 1024–1033. [Link to the article].[/note].
→ An important note in advance:
Ketogenic nutritional concepts serve only to support own therapy proposed by a treating oncologist. By choosing foodstuffs rich in energy and nutrients, they are to help to strengthen the physical condition of the patient and to improve the tolerance as well as the treatment success of the applied method of treatment. In contrast to many dubious and frequently criticised „cancer diets“, the ketogenic diet for cancer does NOT claim to cure cancer and does NOT pretend to render conventional treatment approaches such as chemotherapy and radiotherapy superfluous.
What does ketogenic nutrition mean?
Typical of ketogenic nutrition is that it contains a very high fat – and a very low carbohydrate content. Expressed in numbers, it boils down to the fact that about 75-80% of the supplied energy originates from fat, about 15-20% from protein and only about 5% from carbohydrates.
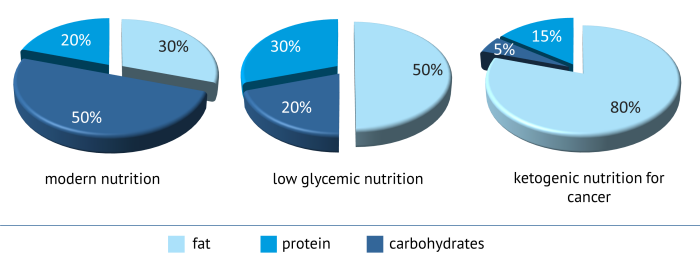 |
| Nutrient distribution of various forms of nutrition |
|---|
The word “ketogen” logically means “ketone body forming”, which makes out the essential part of this kind of nutrition. Ketone bodies such as acetoacetate, beta-hydroxybutyrate and acetone are descendants of fat, which once constituted a vital source of energy for the body. During phases of food shortage, the body starts to break down its storage of fatty tissue. The fats end up in the liver, are converted into ketone bodies and they are then released in the bloodstream to supply the brain, in particular. In this way, humans can survive for some time during periods of starvation. Because of the high carbohydrate supply of our modern diets, healthy people can only achieve such metabolism known as ketosis through fasting.
→ Ketosis through ketogenic nutrition
By following a ketogenic nutrition, it is also possible to achieve a ketogenic metabolism without renouncing food. Due to the very low carbohydrate or sugar intake, the blood glucose levels do not increase and thus no additional insulin is released. This hormone is usually the signal transmitter which allows the body to store the absorbed fats in the fat cells. Without this signal, the body is forced to use the supplied fats directly and to partly convert them into ketone bodies.
→ Clinically proven
The principle, namely to achieve a metabolic state equal to fasting through ketogenic nutrition, is a clinically proven fact in the case of epilepsy patients for decades now. After physicians recognised the soothing effect of fasting on the frequency of seizures, oil and protein nutrition as a recognized method of treatment had already established itself until the introduction of the first epilepsy medications were introduced. Even today, a ketogenic diet remains a medication of choice for patients with epilepsy who do not respond to any of the current medicines.
Also in other clinical pictures, the therapeutic use of ketogenic nutrition is now being discussed: these are neurodegenerative diseases such as Alzheimer’s, Parkinson’s [note]Baranano, K. W.; Hartman, A. L. (2008): The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol 10 (6): 410–419. [Link to the article].[/note] and ALS [note]Zhao, Z. et al. (2006): A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci 7: 29. [Link to the article].[/note], neurological disorders such as autism [note]Evangeliou, A. et al. (2003): Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol 18 (2): 113–118. [Link to the article].[/note] or metabolic disorders such as the polycystic ovary syndrome [note]Mavropoulos, J. C. et al. (2005): The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond) 2: 35. [Link to the article].[/note] or type 2 diabetes [note]Westman, E. C. et al. (2008): The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond) 5: 36. [Link to the article].[/note].
From the hypothesis to the nutrition concept
Already in 1924, the German biochemist and Nobel laureate Otto Warburg recognised the special energy extraction of cancer cells. In his examination of healthy and abnormal cells, he discovered unusually high concentrations of lactic acid (lactate) in the cancer cells – even then, when he exposed the cells to oxygen [note]Warburg, Otto (1924): Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften 12 (50): 1131–1137. [Link to the article].[/note]. This was unusual because most healthy cells produce this only due to lack of oxygen, in other words under anaerobic conditions.
Lactic acid is produced during the fermentation of sugar (anaerobic glycolysis). This energy extraction path is rather ineffective, seen energetically, as it actually requires no oxygen. For most cells, this path is a kind of “emergency solution“, in the case of a temporary lack of oxygen – like for example what happens during intensive training in muscle cells – when it is necessary to produce energy. Once when oxygen becomes available again, then healthy cells return to the usual energy extraction via the cell’s own „power stations“, the mitochondria.
Warburg suspected that the cancer cell mitochondria need to be disrupted and formulated the hypothesis that therein lays the cause of cancer illnesses. An important indication for him of this was also the observation that aggressively growing cancer cells show the altered energy metabolism as opposed to benign tumours that do not. At the latest, with the clarification of the structure of DNA in the 50s and the awareness of carcinogenic genetic modifications, Warburg’s hypothesis of damaged mitochondria as carcinogens, sadly, was ignored and forgotten.
The Warburg effect, namely the fermentation of sugar despite oxygen, called “aerobic glycolysis” by Warburg, was confirmed by several research groups in recent years [note]Langbein, S. et al. (2006): Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer 94 (4): 578–585. [Link to the article].[/note] [note]Schulz, T. J. et al. (2006): Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J Biol Chem 281 (2): 977–981. [Link to the article].[/note]. And also Warburg’s surmise that herein could be concealed an important starting point for cancer therapy, recently proves to be correct in more and more studies. Likewise, it is nowadays considered indisputable that malignant tumours consume high levels of glucose. This particular feature is already used diagnostically in positron emission tomography (PET), during which weak radio-labelled glucose is injected into the patient, which then accumulates in strong glucose-dependent tissues to become visible during imaging. In this way, the radiologist can locate possible cancer foci.
→ Influence factor nutrition
Today we know that certain dietary factors such as nitrosamines in salted meat or mould toxins on rancid nuts can cause carcinogenic DNA damage. Likewise, the majority of physicians are sure that specific food ingredients such as certain vitamins, trace minerals and secondary plant substances can counteract the development of cancer. Nevertheless, it is also becoming increasingly clear that alongside genetic components, cancer also has a strong metabolic component. Thus, not only individual substances that interact with the genetic material, the DNA, play a role. Also the entire nutrition, which exerts a strong influence in its entirety on our metabolism, influences the origin, development and treatment of cancer. Realising this, the consideration to specifically affect metabolic processes that are important for the growth and spread of cancer cells through ketogenic nutrition wins field.
Cell respiration: Energy generation during sufficient oxygen
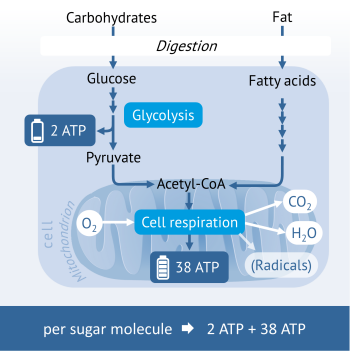 The energy generation of healthy cells takes place in the own „power plants” of cells, namely the mitochondria. Sugar, fats and some amino acids (protein building blocks) are broken down first to intermediates, which are subsequently loaded and then utilised for the formation of the energy carrier ATP in the mitochondria.
The energy generation of healthy cells takes place in the own „power plants” of cells, namely the mitochondria. Sugar, fats and some amino acids (protein building blocks) are broken down first to intermediates, which are subsequently loaded and then utilised for the formation of the energy carrier ATP in the mitochondria.
Sugar such as glucose is broken down through a process called glycolysis to pyruvate. The interesting thing about sugar degradation is that already 2 molecules of the energy carrier ATP incurred outside the mitochondria.
Pyruvate then enters the mitochondria and is there converted into acetyl CoA. Acetyl CoA is a central intermediate in the energy metabolism, which, in the following “cellular respiration”, undergoes two multi-tiered metabolic processes (citric acid cycle and oxidative phosphorylation) at the end of which the formation of the energy carrier ATP takes place. For this last stage, oxygen is required which, during the cell respiration, oxidises to carbon dioxide (CO2) and water (H2O).
Under certain circumstances this last step does not unfold as planned, so that, instead, radicals such as hydrogen peroxide (H2O2) emerge.
Anaerobic Glycolysis: Energy generation during lack of oxygen
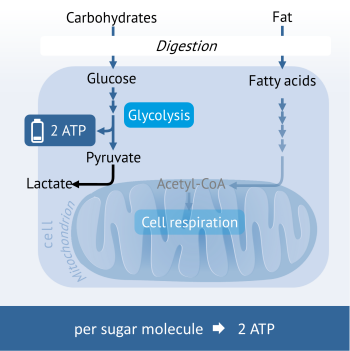 In the case of the temporary shortage of oxygen, the last step of ATP formation cannot be implemented in the mitochondria. This happens for example in healthy muscle cells during intensive physical exercise when more oxygen is consumed for the production of energy than what is delivered through the bloodstream. To continue maintaining the energy generation, the cells use the ATP-forming step of glycolysis. Because the continued processing of the pyruvate in the mitochondria is not possible, this is converted into lactic acid (lactate) instead.
In the case of the temporary shortage of oxygen, the last step of ATP formation cannot be implemented in the mitochondria. This happens for example in healthy muscle cells during intensive physical exercise when more oxygen is consumed for the production of energy than what is delivered through the bloodstream. To continue maintaining the energy generation, the cells use the ATP-forming step of glycolysis. Because the continued processing of the pyruvate in the mitochondria is not possible, this is converted into lactic acid (lactate) instead.
Because this path of energy generation is very inefficient (per sugar molecule only 2 ATP molecules are produced), cells rapidly change back to cell respiration when oxygen is available.
Aerobic glycolysis: Energy generation of aggressive cancer cells
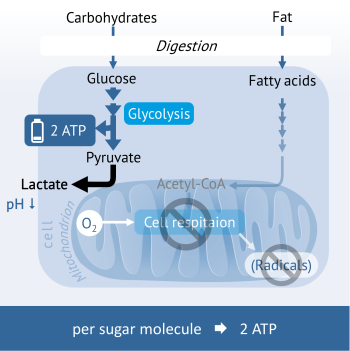 Fermenting cancer cells show an energy metabolism of the anaerobic glycolysis also in the presence of oxygen (also under aerobic conditions). Since only 2 ATP are formed during the breaking down of carbohydrate per glucose unit, the cancer cells are dependent upon a high intake of carbohydrates to cover their immense energy needs.
Fermenting cancer cells show an energy metabolism of the anaerobic glycolysis also in the presence of oxygen (also under aerobic conditions). Since only 2 ATP are formed during the breaking down of carbohydrate per glucose unit, the cancer cells are dependent upon a high intake of carbohydrates to cover their immense energy needs.
Simultaneously, enormous amounts of lactic acid (lactate) are incurred that are transported to the outside to protect the cancer cell. A portion of the lactate reaches the liver, where it is converted to glucose, which the cancer cells in turn can utilise as an energy source. Another part creates a harmful acidic milieu around the tumour tissue that destroys the surrounding tissues on the one hand and at the same time protects cancer cells from attack by immune cells.
Survival advantage fermentation of sugar
The switch-over to the fermentation of sugar means a low energy yield per glucose unit, requires therefore large amounts of sugar, makes cancer cells dependent on one energy source and simultaneously produces high amounts of cell-damaging lactic acid. Why do cancer cells then nevertheless use this unfavourable appearing energy path? The answer lies in some significant survival advantages which the aerobic glycolysis carries along with it:
- Sufficient energy generation in low-oxygen interior of the tumour: The larger the tumour grows, the less oxygen is supplied to the inner situated tumour cells. Glucose can, however, still easily penetrate to the inside and be used as an energy source by these blood-vessel-distant cells.
- Fast energy deployment: The growth of cancer cells requires readily available energy. Because of the dependence on energy, the energy supply through the mitochondria is however limited and is carried out only to the extent in which oxygen is made available. The production of energy through the fermentation of sugar is only dependent on the availability of sugar, which, however, because of our carbohydrate-rich diet, does not pose a limiting factor to the cancer cell.
- Protection against the formation of radicals: During the energy generation in the mitochondria, from time to time reactive oxygen radicals (ROS) are formed as by-products and consequently cell-damaging oxidative stress is caused. The high energy demand of cancer cells would greatly speed up this process and contribute rapidly to their downfall. The fermentation of sugar enables the energy production without the cell-damaging formation of radicals.
- Protective lactate shell and matrix degeneration: During the fermentation of sugar, high levels of lactate (lactic acid) are transported to the outside of the cells as a protective measure. The tumour is surrounded by an acidic environment to such an extent that it destroys the surrounding tissue (matrix degeneration) and thus gives way to the invasive spread of cancer cells. At the same time, the lactate shell protects scattering cancer cells against attack by immune cells in the bloodstream.
- Therapy resistance: Through the “switching off” of the mitochondria, the fermenting cancer cells also protect themselves against therapies, which take aim at the increased formation of radicals (radiation) or to deliver the knock-out blow , namely the death of the cells, the apoptosis (chemotherapy) [note]Xu, R. H. et al. (2005): Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65 (2): 613–621. [Link to the article].[/note] [note]Vaughn, A. E.; Deshmukh, M. (2008): Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol 10 (12): 1477–1483. [Link to the article].[/note].
Growth factor insulin
Sugar, nevertheless, does not only play a role as preferred source of energy of fermenting cells in the development of cancer. Elevated blood sugar levels already encourage the emergence of cancer. Responsible for this is the hormone insulin, which is secreted by each sugar- or starch-rich meal to reduce blood sugar level and return it back to a normal level again. Insulin itself, but also the insulin-like growth factor IGF-1 act as growth promoters, which promotes the growth, distribution and metastasis of tumour cells [note]Pollak, M. (2008): Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8 (12): 915–928. [Link to the article].[/note] [note]Gallagher, E. J.; LeRoith, D. (2011): Minireview: IGF, Insulin, and Cancer. Endocrinology 152 (7): 2546–2551. [Link to the article].[/note] in cell experiments. This is specifically the case when the insulin levels are constantly high in the blood as in the case of type 2 diabetes. People with diabetes are at an increased risk of certain cancers such as breast, uterus, colon, stomach, liver, bladder and pancreatic cancer [note]Hua, F.; Yu, J. J.; Hu, Z. W. (2016): Diabetes and cancer, common threads and missing links. Cancer Lett 374 (1): 54–61. [Link to the article].[/note]. The probability that a diabetic can contract for example breast or colon cancer is about 23% to 26% more likely than for non-diabetics [note]Bruijn, K. M. de et al. (2013): Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg 100 (11): 1421–1429. [Link to the article].[/note]. Also independent of a diabetes type, various studies indicate that a high carbohydrate diet increases the risk of cancer [note]Gnagnarella, P. et al. (2008): Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr 87 (6): 1793–1801. [Link to the article].[/note]. Already for the prevention of cancer, it is sensible to keep carbohydrate intake moderate and to avoid high sugar peaks followed by a high insulin release. Also during follow-up care, carbohydrate diet continues to play a role. In this way, scientists could provide evidence that for example a high-carbohydrate diet increases the relapse rate in colorectal cancer patients while the chance of survival decreases [note]Meyerhardt, J. A. et al. (2012): Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst 104 (22): 1702–1711. [Link to the article].[/note]. Women, whose breast cancer was positive for the IGF receptor, increase their relapse rate through a high-glycemic, carbohydrate diet by as much as 500% [note]Emond, J. A. et al. (2014): Risk of breast cancer recurrence associated with carbohydrate intake and tissue expression of IGFI receptor. Cancer Epidemiol Biomarkers Prev 23 (7): 1273–1279. [Link to the article].[/note].
Ketogenic nutrition instead of fasting
Isolated case reports of patients, who fasted for some days before chemotherapy or radiation therapy, repeatedly indicate an improved tolerability for the cancer therapy with, for example, less fatigue, nausea, gastrointestinal discomfort and headache [note]Safdie, F. M. et al. (2009): Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 1 (12): 988–1007. [Link to the article].[/note]. Mouse and cell experiments indicate that low blood sugar levels and lower levels of IGF – 1 due to fasting, play an important role here [note]Raffaghello, Lizzia et al. (2010): Fasting and differential chemotherapy protection in patients. Cell Cycle 9 (22): 4474–4476. [Link to the article].[/note].
But, fasting is not suited for all cancer patients and carries the risk of losing even more bodily substances, which are important for survival. A ketogenic nutrition is able to reduce blood sugar, as well as IGF-1 levels, to adapt the healthy cells to a metabolism based on fasting and to still provide the body with energy, protein and micro-nutrients.
Is there any evidence of effectiveness thus far?
Many critics of the ketogenic diet concepts complain that there are too few studies on the efficacy in humans. It is true that many findings on the effects of sugar deprivation, for example the inhibition of fermentation metabolism in cancer growth and the therapeutic sensitivity come from cell- and animal experiments (we have summarised some of these studies: Reference list).
A fundamental problem with human studies is, however: they cost a lot of money. After all, which big company has interest to invest money towards researching nutritional approaches, which is theoretically achievable with simple foodstuffs? Unfortunately none, so that we have been forced to fall back on positive case reports from practice and the results of clinics who implement fasting or ketogenic diets for their patients. But the trend currently shows that, because of the growing number of promising findings from cell and animal experiments, more and more research groups and clinics are ready to carry out their own small trials on patients.
First studies on tolerance and efficacy on patients:
- Schweinfurt Hospital: Six patients independently nourished themselves ketogenic, while they underwent radiation. Four of them achieved sufficient ketone body levels. All patients lost weight, however, only in fat but not in muscle mass. The overall condition during the radiation was overall very positive and all felt happy with the diet. Five of the patients reached, as expected, a regression of the tumour. Only one patient with metastatic small-cell lung cancer showed a slight progress in health, which sadly progressed quicker when the diet was stopped [note]Klement, Rainer J.; Sweeney, Reinhart A. (2016): Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Research Notes 9 (1): 143. [Link to the article].[/note].
- ERGO study: The study by the University Clinic in Tubingen examined the impact of ketogenic nutrition in 20 patients with recurring glioblastoma (incurable form of brain tumour, which is difficult to treat). During the treatment, there were no serious side effects. In the findings, the ketogenic diet was assessed as feasible and safe, which is useful only as a supportive therapy option [note]Rieger, Johannes et al. (2014): ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol 44 (6): 1843–1852. [Link to the article].[/note].
- University Clinic Wurzburg: The University Clinic Wurzburg offers patients the possibility to follow an oil and protein-weighted diet in addition to the standard treatment. Patients, who were able to implement the 3-month treatment with the ketogenic food showed some improvements with regard to their quality of life and some blood parameters [note]Schmidt, M. et al. (2011): Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond) 8: 54. [Link to the article].[/note].
- University of Pittsburgh, USA: The researchers analysed the data of 53 patients with glioblastoma, of which, during the treatment, six followed a ketogenic diet. The diet was well tolerated and the side effects of cancer therapy were relatively small. In comparison to other patients, the blood glucose levels of patients on ketogenic nutrition, in spite of steroid therapy, were in the normal range [note]Champ, C. E. et al. (2014): Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol 117 (1): 125–131. [Link to the article].[/note].
- University of Leiden, Netherlands: Thirteen patients with Her2 negative breast cancer were examined, 7 of which fasted a day before and after chemotherapy. The chemotherapy had less of an impact on their blood levels in comparison to the women who did not fast. Especially the red blood cells and platelet levels were significantly better [note]Groot, Stefanie de et al. (2015): The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer 15: 652. [Link to the article].[/note].
Why is it worth a try?
A low-carbohydrate diet has a number of advantages for the patient, also despite a direct anti-cancer effect:
- Sufficient energy due to a high fat content,
- Supply of important protein components to preserve the body’s substance, the immune system and metabolic performance,
- Absorption of important vitamins and minerals such as bio-active herbal ingredients through low-carbohydrate vegetables, berries, nuts, herbs and spices,
- Supply of anti-inflammatory fatty acids through high-quality vegetable oils, nuts and fatty cold-water fish,
- Reduction of cancer risk factors by lowering blood sugar and insulin levels as well as reduction of overweight and inflammation-promoting body fat.
Because the body is adapted to be able to live with a low carbohydrate intake, long-term side effects of a carbohydrate reduction are unlikely.
Last but not least, the option to support the own therapy with the help of a nutrition that is adapted to the special features of tumour metabolism gave many cancer patients the feeling that they themselves are contributing actively to their own recovery. This has an enormous psychological effect which is not to be underestimated for the cancer therapy.
Sources

